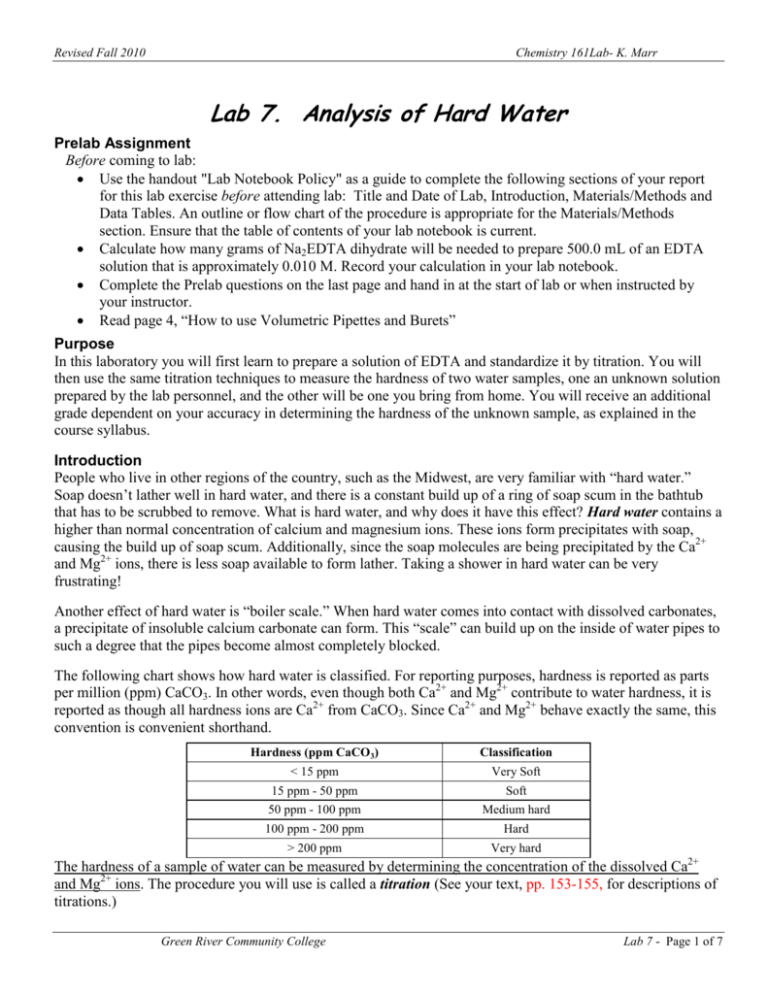
Determination Of Hardness Of Water Lab Report - Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The document describes an experiment to determine the hardness of water samples through titration with edta using indicators. The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows:
Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The document describes an experiment to determine the hardness of water samples through titration with edta using indicators. The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows:
The document describes an experiment to determine the hardness of water samples through titration with edta using indicators. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows:
Analysis of Hard Water Lab
Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows: The document describes an experiment to determine the hardness of water samples through titration with edta using indicators.
Lab Report Complexometric Determination of Water Hardness StudyMoose
The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows: Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The document describes an experiment to determine the hardness of water samples through titration with edta using indicators.
SOLUTION Experiment on determination of total hardness of supplied
Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows: The document describes an experiment to determine the hardness of water samples through titration with edta using indicators.
Determination of water hardness lab report example 144 Words
The document describes an experiment to determine the hardness of water samples through titration with edta using indicators. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows:
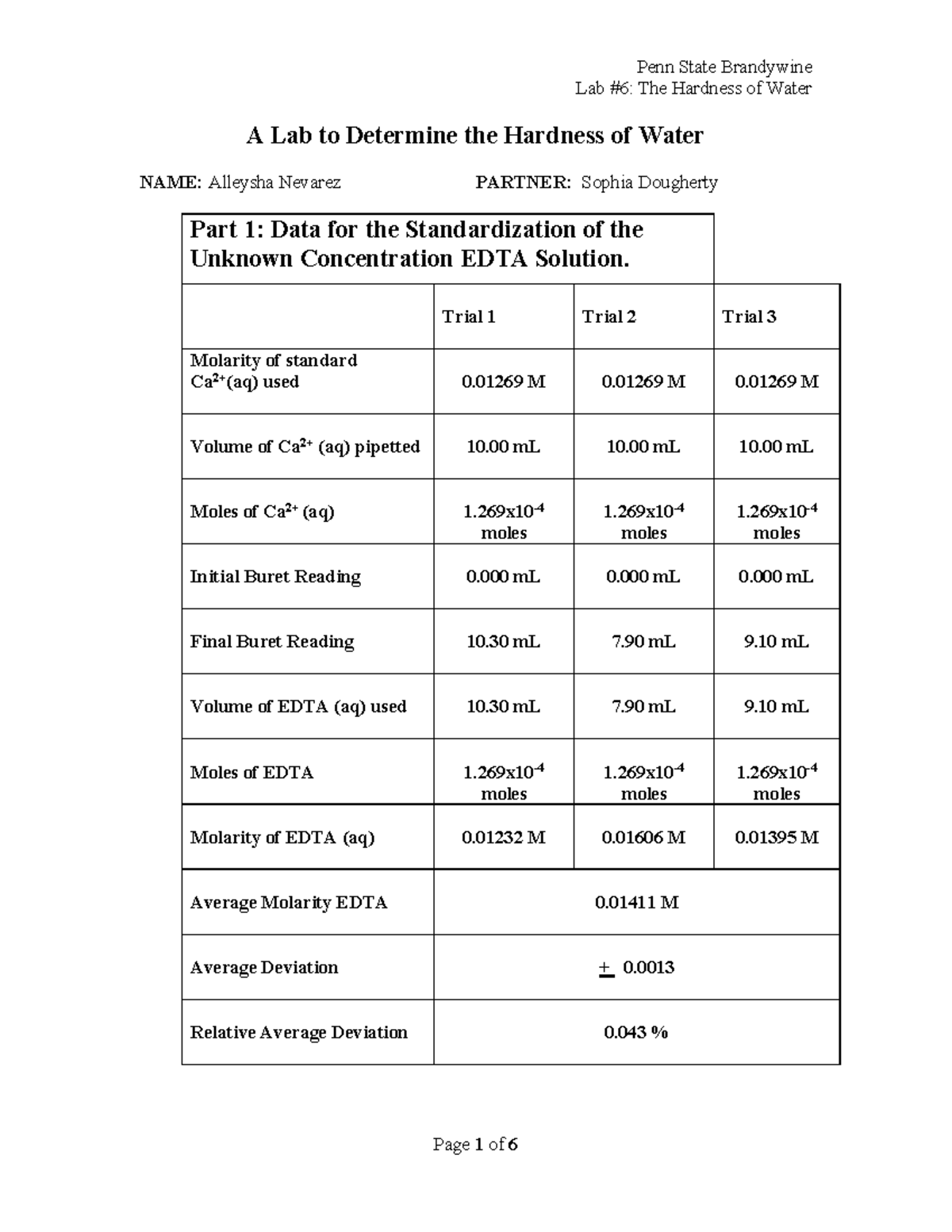
Hardness of Water LAB Report Lab 6 The Hardness of Water A Lab to
The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows: Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The document describes an experiment to determine the hardness of water samples through titration with edta using indicators.
Experiment 3 (Determination of Hardness in Water) Subject
Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows: The document describes an experiment to determine the hardness of water samples through titration with edta using indicators.
Determination of Water Hardness Report Download Free PDF Titration
Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The document describes an experiment to determine the hardness of water samples through titration with edta using indicators. The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows:
Determination of Hardness of Water_A_ Complete Procedure (ASTM D1126
The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows: Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The document describes an experiment to determine the hardness of water samples through titration with edta using indicators.
SOLUTION Experiment on determination of hardness of water Studypool
The document describes an experiment to determine the hardness of water samples through titration with edta using indicators. The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows: Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid).
CHM 112 Hardness of Water Analysis Report Lab Report CHM112 NVCC
The document describes an experiment to determine the hardness of water samples through titration with edta using indicators. The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows: Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid).
Water Hardness Can Be Readily Determined By Titration With The Chelating Agent Edta (Ethylenediaminetetraacetic Acid).
The document describes an experiment to determine the hardness of water samples through titration with edta using indicators. The degree of hardness of drinking water has been classified in terms of the equivalent caco3 concentration as follows: