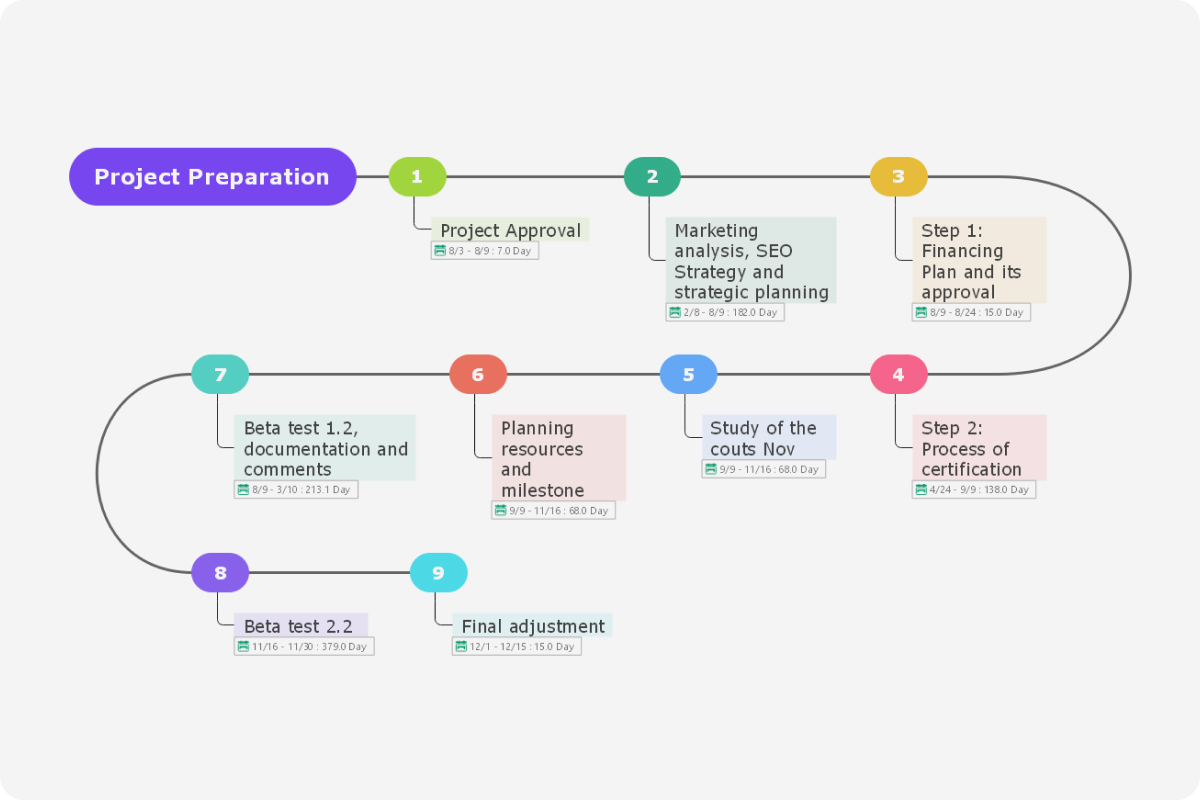
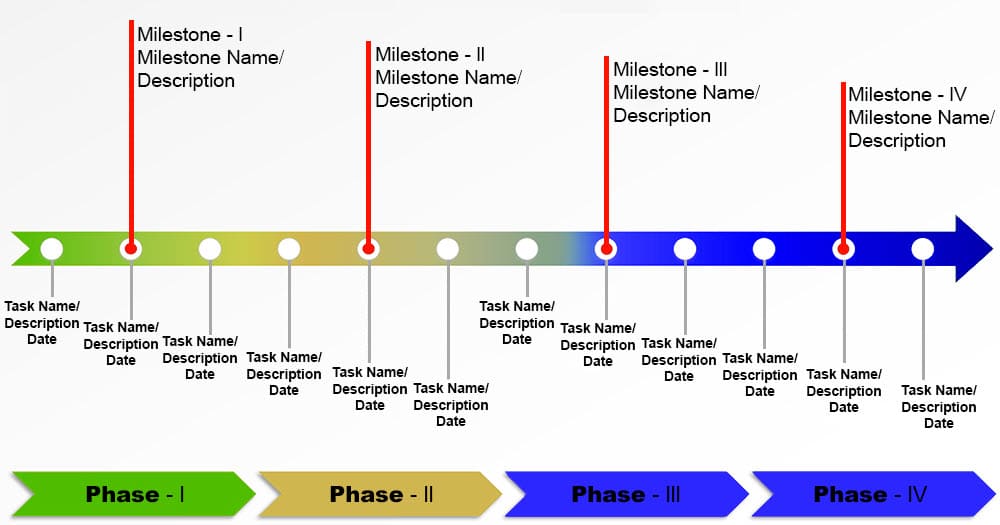
Milestone Plan Nih - This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator. In addition, a timeline (or gantt chart) including milestones is required for all applications. Milestones may include, as applicable, but are not limited to: Use your era commons username and password to. Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. Milestones should be easily measurable and realistic. Go to the nimh recruitment milestone reporting site at:
In addition, a timeline (or gantt chart) including milestones is required for all applications. Milestones should be easily measurable and realistic. Go to the nimh recruitment milestone reporting site at: Use your era commons username and password to. Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. Milestones may include, as applicable, but are not limited to: This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator.
Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. Go to the nimh recruitment milestone reporting site at: Use your era commons username and password to. Milestones may include, as applicable, but are not limited to: Milestones should be easily measurable and realistic. In addition, a timeline (or gantt chart) including milestones is required for all applications. This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator.
Top 35 Timeline And Milestone Templates to Keep An Event on Track The
This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator. Go to the nimh recruitment milestone reporting site at: Milestones should be easily measurable and realistic. Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. Use your era commons username and password to.
Project Milestone Sampletemplates vrogue.co
Use your era commons username and password to. Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. Milestones should be easily measurable and realistic. In addition, a timeline (or gantt chart) including milestones is required for all applications. Go to the nimh recruitment milestone reporting site at:
Milestone Charts 101 With Samples and Templates
Milestones should be easily measurable and realistic. This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator. Use your era commons username and password to. Go to the nimh recruitment milestone reporting site at: Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial.
High Level Milestone Plan Template
This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator. Milestones should be easily measurable and realistic. Go to the nimh recruitment milestone reporting site at: Milestones may include, as applicable, but are not limited to: Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial.
Free Milestone Templates & Examples EdrawMind
Milestones should be easily measurable and realistic. In addition, a timeline (or gantt chart) including milestones is required for all applications. Milestones may include, as applicable, but are not limited to: Go to the nimh recruitment milestone reporting site at: This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator.
Milestone Plan Template
This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator. Milestones may include, as applicable, but are not limited to: In addition, a timeline (or gantt chart) including milestones is required for all applications. Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. Use your era commons username.
What is a Milestone Schedule? Definition and Example PM Study Circle
In addition, a timeline (or gantt chart) including milestones is required for all applications. Milestones should be easily measurable and realistic. Use your era commons username and password to. Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. Go to the nimh recruitment milestone reporting site at:
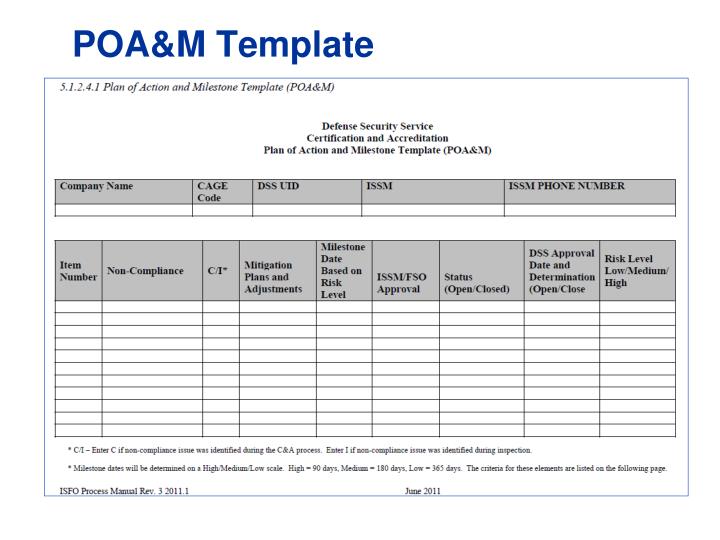
Plan Of Action And Milestones Template
Go to the nimh recruitment milestone reporting site at: Use your era commons username and password to. Milestones may include, as applicable, but are not limited to: Milestones should be easily measurable and realistic. In addition, a timeline (or gantt chart) including milestones is required for all applications.
Future Directions in NIDCD Research 20232027 NIDCD Strategic Plan
Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. Go to the nimh recruitment milestone reporting site at: This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator. Milestones should be easily measurable and realistic. Milestones may include, as applicable, but are not limited to:
Top 10 Project Milestone Templates with Samples and Examples
Use your era commons username and password to. Milestones should be easily measurable and realistic. Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial. Milestones may include, as applicable, but are not limited to: In addition, a timeline (or gantt chart) including milestones is required for all applications.
Milestones May Include, As Applicable, But Are Not Limited To:
Go to the nimh recruitment milestone reporting site at: Milestones should be easily measurable and realistic. In addition, a timeline (or gantt chart) including milestones is required for all applications. Timeline for a typical milestone plan based on the assessed risk rating of the clinical trial.
Use Your Era Commons Username And Password To.
This funding plan is developed by nih in collaboration with the cooperative agreement principal investigator.