Permissable Milestone Payments Clinical - For example, milestones are often structured to be payable upon the initiation of a clinical trial, the regulatory approval of a. In direct payments, large pharma companies prefer to pay investigators in milestones. Such schedules usually provide for periodic payments at regular intervals or payments upon the completion of milestones (e.g., enrollment. In these instances, the “budget” presents itself as a binding legal document with terms that could have unforeseen. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. On average, the initial payment can.
In these instances, the “budget” presents itself as a binding legal document with terms that could have unforeseen. In direct payments, large pharma companies prefer to pay investigators in milestones. On average, the initial payment can. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. For example, milestones are often structured to be payable upon the initiation of a clinical trial, the regulatory approval of a. Such schedules usually provide for periodic payments at regular intervals or payments upon the completion of milestones (e.g., enrollment.
In these instances, the “budget” presents itself as a binding legal document with terms that could have unforeseen. On average, the initial payment can. Such schedules usually provide for periodic payments at regular intervals or payments upon the completion of milestones (e.g., enrollment. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. For example, milestones are often structured to be payable upon the initiation of a clinical trial, the regulatory approval of a. In direct payments, large pharma companies prefer to pay investigators in milestones.
Introduction to Billing PeopleSoft Grants ppt download
In direct payments, large pharma companies prefer to pay investigators in milestones. For example, milestones are often structured to be payable upon the initiation of a clinical trial, the regulatory approval of a. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. On average, the.
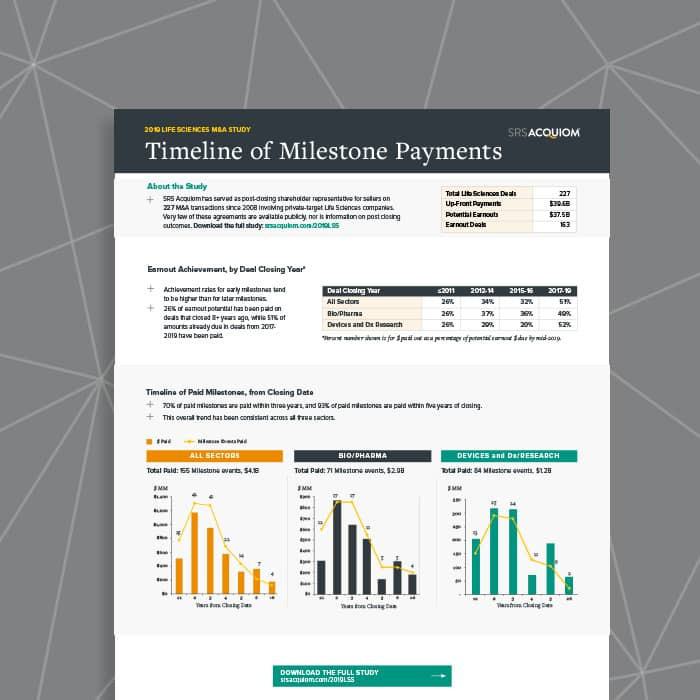
Life Sciences Milestone Payments from Closing Date SRS Acquiom
On average, the initial payment can. Such schedules usually provide for periodic payments at regular intervals or payments upon the completion of milestones (e.g., enrollment. For example, milestones are often structured to be payable upon the initiation of a clinical trial, the regulatory approval of a. In direct payments, large pharma companies prefer to pay investigators in milestones. As part.
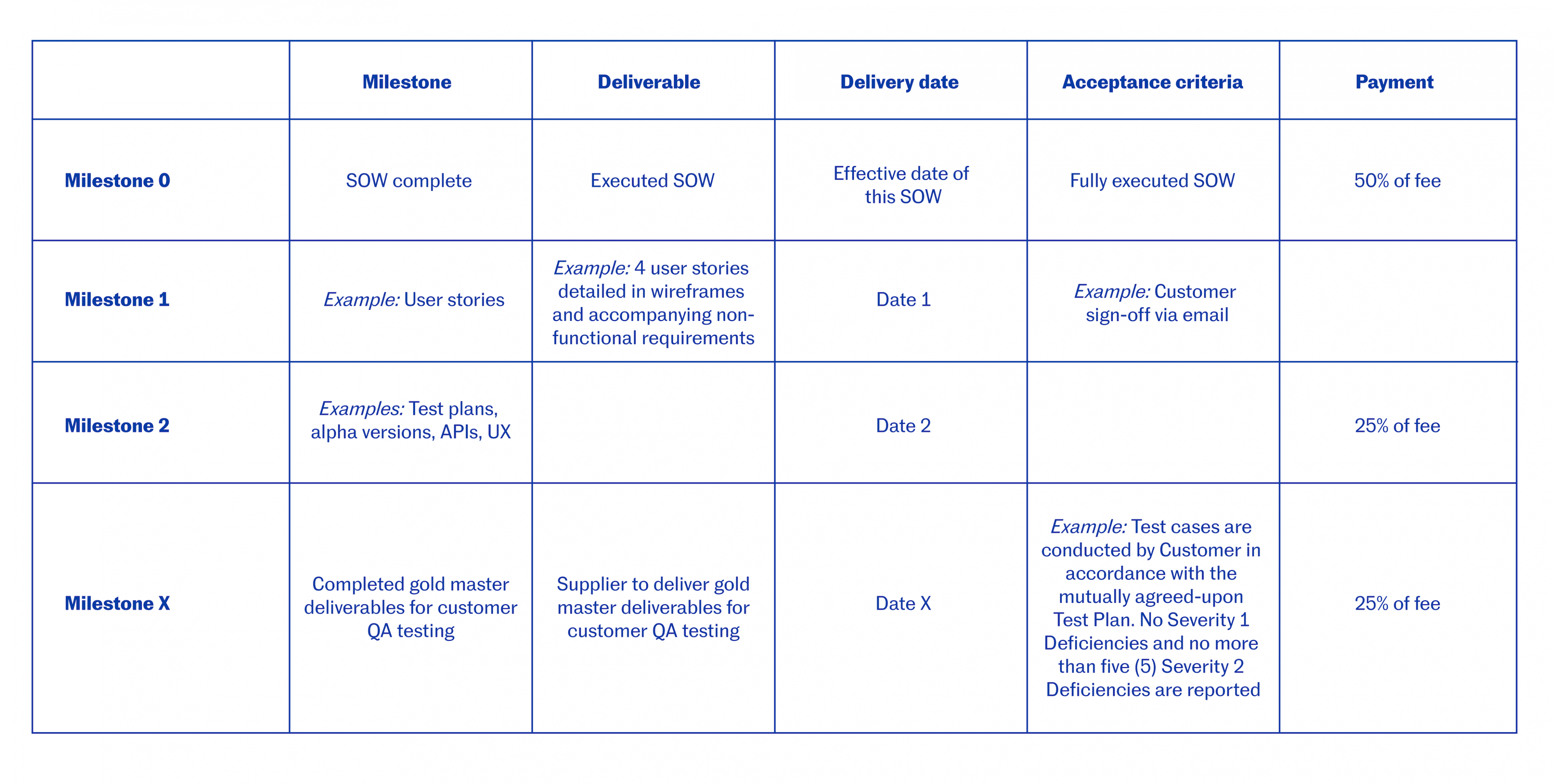
Milestone Payment Schedule Template
For example, milestones are often structured to be payable upon the initiation of a clinical trial, the regulatory approval of a. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. On average, the initial payment can. Such schedules usually provide for periodic payments at regular.
PPT Clinical Trials 1 PowerPoint Presentation, free download ID1670302
On average, the initial payment can. In these instances, the “budget” presents itself as a binding legal document with terms that could have unforeseen. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. For example, milestones are often structured to be payable upon the initiation.
New based Payment System ppt video online download
On average, the initial payment can. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. In these instances, the “budget” presents itself as a binding legal document with terms that could have unforeseen. In direct payments, large pharma companies prefer to pay investigators in milestones..
Value Partnerships Enterprise Services Siemens Healthineers Siemens
On average, the initial payment can. For example, milestones are often structured to be payable upon the initiation of a clinical trial, the regulatory approval of a. Such schedules usually provide for periodic payments at regular intervals or payments upon the completion of milestones (e.g., enrollment. In these instances, the “budget” presents itself as a binding legal document with terms.
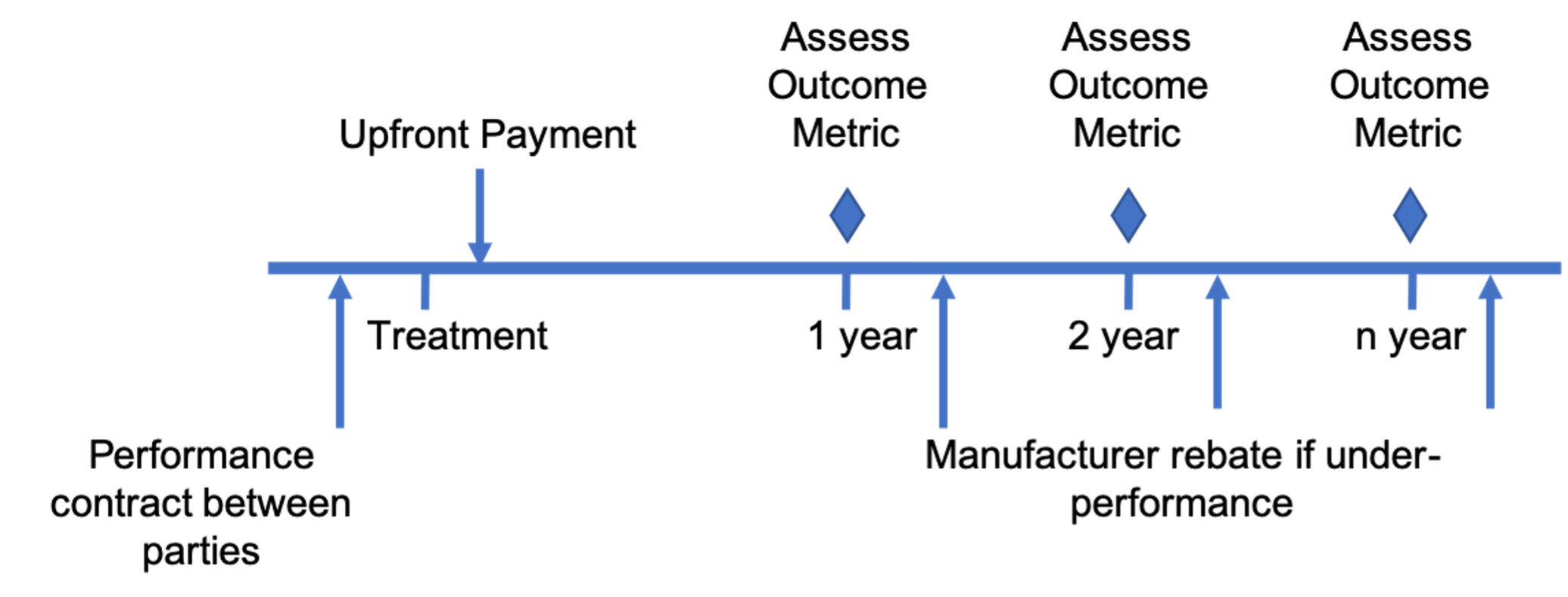
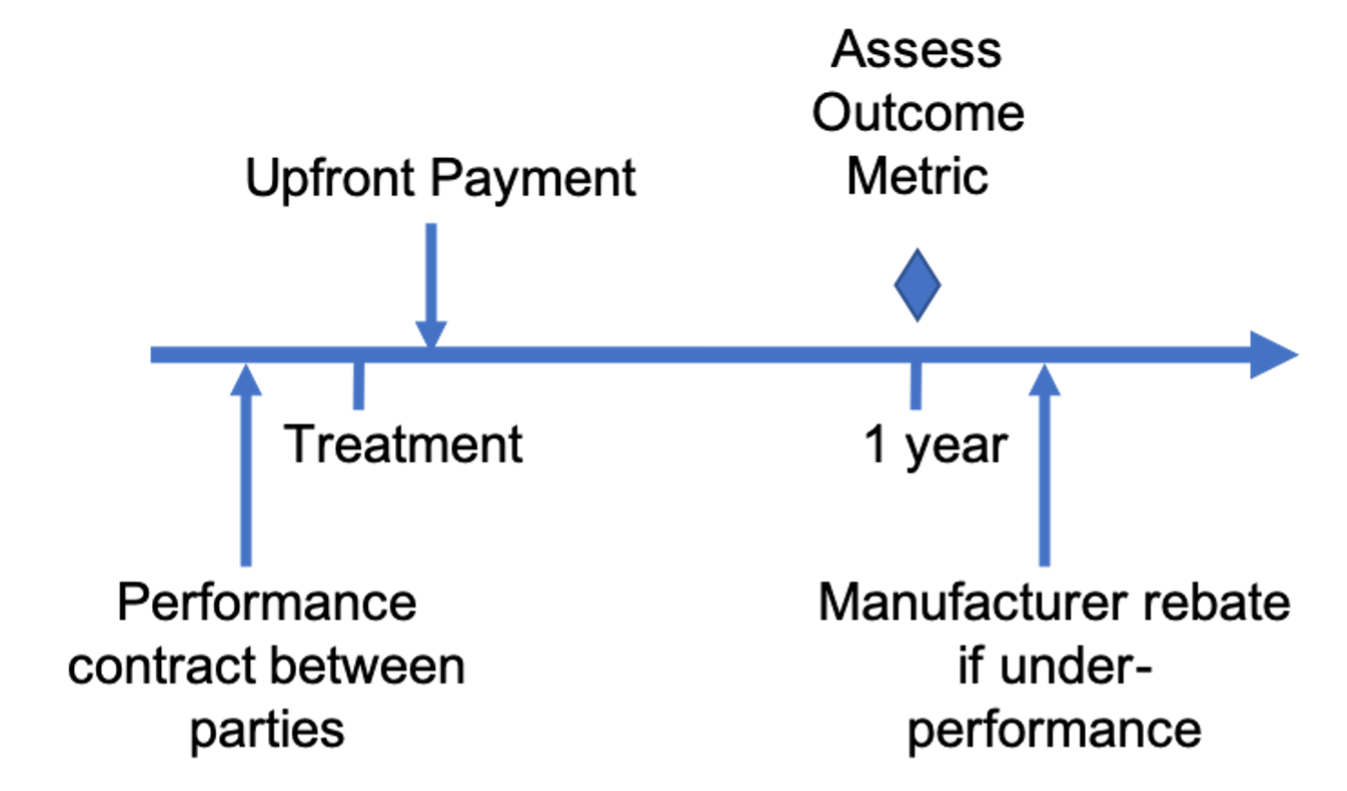
Multiyear milestonebased contracts Center for Biomedical System Design
Such schedules usually provide for periodic payments at regular intervals or payments upon the completion of milestones (e.g., enrollment. In these instances, the “budget” presents itself as a binding legal document with terms that could have unforeseen. On average, the initial payment can. For example, milestones are often structured to be payable upon the initiation of a clinical trial, the.
Milestonebased contracts Center for Biomedical System Design
As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. In these instances, the “budget” presents itself as a binding legal document with terms that could have unforeseen. On average, the initial payment can. In direct payments, large pharma companies prefer to pay investigators in milestones..
How to Use Milestone Payments to Improve the Right Way
In these instances, the “budget” presents itself as a binding legal document with terms that could have unforeseen. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. On average, the initial payment can. For example, milestones are often structured to be payable upon the initiation.
Milestones and Deliverables Population Health Management Learning Center
On average, the initial payment can. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. For example, milestones are often structured to be payable upon the initiation of a clinical trial, the regulatory approval of a. Such schedules usually provide for periodic payments at regular.
In These Instances, The “Budget” Presents Itself As A Binding Legal Document With Terms That Could Have Unforeseen.
In direct payments, large pharma companies prefer to pay investigators in milestones. As part of this process, nichd applications to be funded or awards with one or more clinical trials must establish milestones and provide post. For example, milestones are often structured to be payable upon the initiation of a clinical trial, the regulatory approval of a. On average, the initial payment can.